How to Take Out a Virus: Spotlight on NETosis
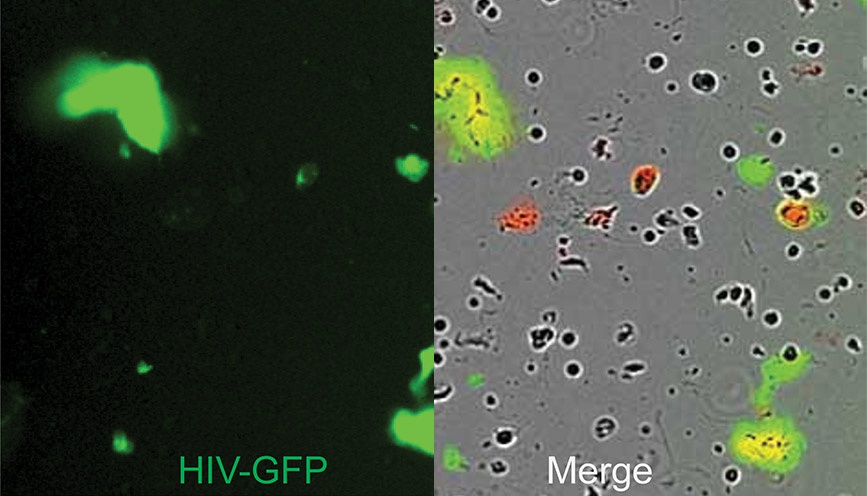
Neutrophils isolated from the female genital tract cultured in media with impermeant DNA dye (red) and stimulated with GFP-labeled HIV viral-like particles (green) to induce NET formation. Left panel shows the GFP-labeled viral-like particles entrapped in NETs. Right panel shows the merge image of phase contrast and fluorescent signals, with HIV trapped in the NETs appearing as green/yellow. - Image courtesy of Dr. Marta Rodriguez-Garcia, Tufts University School of Medicine
By Jill Granger, M.S., Technical Writer, Product Management, Sartorius
As COVID-19 lingers on, with vaccines still under development, many are searching for new therapeutic strategies to “take out” this latest virus. One approach is to manipulate one of the immune system’s strategies for protecting the host and removing infected cells: the cell death mechanism of NETosis. Using Incucyte® live-cell imaging and analysis to both capture and analyze the kinetics of this key event in the development of new NETosis assays can provide new insights for therapeutic targeting and management.
In NETosis, neutrophils corner and destroy the invading pathogen through the formation of neutrophil extracellular traps (NETS). These tangled, extracellular, web-like formations contain DNA from neutrophils, nuclear chromatin, granular proteins with antimicrobial properties, as well as oxidant enzymes. NETs are thought to provide a physical barrier, trapping pathogens in their spider-like webs to prevent further infection. This also exposes them to more concentrated antimicrobial proteins near the site of infection, which could also reduce the toxic effects of proteases. NETs are produced when enzymes within the neutrophil are transported to the nucleus, followed by chromatin decondensation. The internal cellular membranes break down, are disrupted, and the NETs released.
Examining NETosis can be challenging due to the similarities with other cell death mechanisms, as well as being technically laborious by standard fluorescence microscopy methods. These can be subject to bias, and are often performed as endpoint assays, thereby missing important kinetic information. To address this issue, Gupta, Chan, and colleagues at the National Institute of Health developed a high-throughput imaging technique using Incucyte technology that not only quantified NETosis, but also distinguished this process from other cell death mechanisms, such as apoptosis and necrosis, through generation of characteristic “fingerprint curves”. These arose from distinct kinetic patterns that were generated with known NET-inducing stimuli.1 This assay was based on observable, distinct changes in nuclear morphology, such as the loss of multi-lobulated nuclei, nuclear condensation, and compromise of membranes. Use of the automated Incucyte platform and software, along with a dual-dye system for DNA staining that was membrane integrity-dependent, enabled the rapid quantification of NETosis by applying filtering by fluorescence intensity and nuclear size. These findings were also confirmed and validated against the predicate fluorescence microscopy method assessing extruded granule proteins bound to DNA or also histones. The assay performed rapid assessment that was more sensitive compared to fluorescence microscopy, unbiased, and reproducible to provide kinetic information that was helpful for the identification of therapeutic targets.
Having the ability to quantify NETosis in real time can provide important insights on how this response can be an effective, first-line defense against a viral pathogen. Dr. Rodriguez-Garcia and colleagues (formerly at Dartmouth, now at Tufts) studied the impact of NET formation by neutrophils at mucosal sites during HIV infection.2 Incucyte live-cell imaging and analysis was used to capture the in vitro dynamics of GFP-tagged HIV-VLP (HIV viral-like particles) entrapped by human genital neutrophils, which had been cultured in cell impermeant DNA-dye (red). When the neutrophils released DNA as part of the NET, this was visualized as red signal, while HIV-VLP had a green signal. HIV-VLP entrapment by the NETs was visualized as co-localization of red (NET) and green (HIV-VLP) signals. They found that NETs were released within minutes of viral exposure. This release was also increased over similarly-treated blood neutrophils, indicating the distinct nature of the mucosal population. Further, this process occurred through reactive oxygen species-independent mechanisms, effectively trapping the HIV-VLPs. Subsequent in vitro analysis of pre-formed NETs with infectious HIV prevented infection through viral inactivation. Until this study, this form of mucosal protection against HIV infection had not been characterized. These insights filled an important gap in the current understanding of early immune defense against HIV in the genital mucosa, which could have preventative implications. We spoke more with Dr. Rodriguez-Garcia to learn how the use of live cell imaging assisted in her research and significance of her findings:
How did live-cell imaging with Incucyte help you capture and understand the unique biology of human genital neutrophils?
“Using the Incucyte platform allowed for the characterization of the very early events of NET formation following viral exposure. Given that the number of neutrophils isolated from human genital tissues can be limiting, the ability to perform time-lapse imaging in a 96-well format was key to understanding the dynamics of NET formation following HIV exposure, and also, for the simultaneous comparison of different genital tissues and treatments to understand the underlying mechanisms of NET formation in the genital tract.“
This study highlights the different responses of neutrophil population within the body. Is it possible that other mucosal areas might have similar NET alterations?
“It is very possible. There is still much to learn about neutrophils in mucosal surfaces and how the tissue environment modifies their functions.”
As with any immune reaction, however, NETosis may be too strong and spin out of control. This can lead to a systemic response with ensuing production of cytokines, chemokines, and immune complexes, giving rise to collateral damage in surrounding tissues and organs, and even death. NETs have been implicated in the inflammatory pathogenesis of diseases such as thrombosis and stroke, preeclampsia, malaria, cancer, and autoimmune conditions. In the case of viral infections, excessive NET formation can pose serious problems for some individuals. In a letter to the Editors of Thrombosis Research in April this year, Drs. Mozzini and Girelli from the University of Verona called for more testing to determine the role of NETosis in COVID-19, along with the possible consideration of a NET-oriented clinical trial.3 Most recently, there has been a clinical report by Zuo, Kanthi, and Knight et al. that demonstrated the presence of cell-free DNA, myeloperoxidase DNA (MPO-DNA), and citrullinated histone H3 (Cit-H3) in COVID-19 patient serum samples. MP0-DNA and Cit-H3 are markers for NET formation, and cell-free DNA correlated clinically with other markers of acute phase disease.4.
Drs. Chirivi, Raats, and colleagues (at ModiQuest B.V.) recently reported on the release of NET into the extracellular environment during inflammation.5 They tested the efficacy of engineered therapeutic anti-citrullinated protein antibodies (tACPA) on NET disease pathology in mouse inflammatory disease models of arthritis, pulmonary fibrosis, colitis, and sepsis. Incucyte live-cell analysis was used to capture images of NET release from neutrophils in the presence of an engineered therapeutic anti-citrullinated protein antibody (tACPA), which bound to citrulline at position3 (Cit3) in histone 2A (citH2A) and 4 (citH4). The antibody showed both therapeutic and prophylactic potential for inflammatory disease associated with NET pathology, being the first in kind described to interfere with NET expulsion into the extracellular space. We touched base with Dr. Raats with some questions about this important discovery:
How did the use of live-cell imaging add to your understanding of the kinetic effects of the tACPA antibody on NET binding and release?
“A good example of this was provided by the study towards the role of Fab2 and Full size antibodies in NET inhibition. Both Fab2 and Full-size antibody inhibited NET formation. The different staining capacities of the live imaging allowed real-time visualization of the NET formation and the subsequent inhibition thereof. This made clear on what point in the NET formation tACPA antibodies bind to the neutrophil and inhibit the NET formation.”
Could this type of antibody have possible application for infectious diseases such as viral infections?
“This antibody could potentially have utility in the treatment of secondary consequences of virus infections. The antibody interferes with NET formation and blocks the effect and promotes clearance of toxic histones that cause tissue damage. Therefore, it may be useful to inhibit or prevent the tissue damaging and thrombus inducing effects of NETs induced by viral infections. However this is currently only a hypothesis and further testing by Citryll B.V. may shed further light on this.”
These studies highlight the need to examine the kinetics of this important cell death mechanism in greater detail for deeper biological insight, which can be readily provided through the use of Incucyte live-cell analysis. Understanding and exploiting the immune system’s “hit-man” potential could go a long way to combatting a diversity of diseases, including our latest viral nemesis.
- Watch a video showing the Incucyte NETosis assay in action
- Incucyte NETosis Assay for Live-Cell Analysis
- Discover more about Incucyte assays for neutrophil function
- Discover more about Incucyte immunology assays
- Discover more about Incucyte virology assays
For further Reading:
- Gupta S, Chan DW, Zaal KJ, Kaplan MJ. A High-Throughput Real-Time Imaging Technique To Quantify NETosis and Distinguish Mechanisms of Cell Death in Human Neutrophils. J Immunol. 2018;200(2):869-879. doi:10.4049/jimmunol.1700905
- Barr FD, Ochsenbauer C, Wira CR, Rodriguez-Garcia M. Neutrophil extracellular traps prevent HIV infection in the female genital tract. Mucosal Immunol. 2018;11(5):1420-1428. doi:10.1038/s41385-018-0045-0
- Mozzini C, Girelli D. The role of Neutrophil Extracellular Traps in Covid-19: Only an hypothesis or a potential new field of research? Thromb Res. 2020;191:26-27.doi:10.1016/j.thromres.2020.04.031
- Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):138999. Published 2020 Jun 4. doi:10.1172/jci.insight.138999
- Chirivi, R.G.S., van Rosmalen, J.W.G., van der Linden, M. et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol (2020). doi.org/10.1038/s41423-020-0381-3
Credits:
Dr. Marta Rodriguez-Garcia, M.D., Ph.D.
Assistant Professor, Tufts University School of Medicine
Dr. Rodriguez-Garcia lab webpage
Dr. Jos Raats, Ph.D.
Managing Director
ModiQuest B.V. / AbSano B.V.
jraats@modiquest.com / jraats@absano.com
www.absano.com / www.citryll.com
