Uncovering the Secrets of Cellular Motility: The Impact of Actin Nt-Acetylation on the Cytoskeleton
An interview with Dr. Henriette Aksnes, University of Bergen.
By Jill Granger, M.S., Technical Writer, Product Management, Sartorius
Cellular motility in animals is a dynamic process, dependent on the interactions of microfilaments (e.g. actin and myosin), intermediate filaments (e.g. keratin), and microtubules. Working together in concert, these cytoskeletal structures engage in cellular activities such as cellular migration, transportation of vesicles, and gene transcription to name a few. In particular, the actin protein is a busy player; shuffling states between polymerization at the leading edge of cells to help pull them forward, and depolymerization at the trailing edge, pushing them from behind. This train-like “chugging along” requires a dynamic interplay of hard-working actin proteins, which transition between monomeric and filamentous forms to sustain cellular activities. This process is regulated not only by actin binding and signaling proteins, but also posttranslational modifications that control cellular functions, particularly N-terminal (Nt)-acetylation. Until recently, the role of Nt-acetylation was not well characterized, and the identity of actin’s N-terminal acetyltransferase (NAT) was unknown. However, since this is one of the most highly conserved actin modifications in animals, it was clearly important: this mystery needed to be solved! Scientists had been puzzling over this question for decades, searching for their own version of a “cellular Titanic”.
Enter the watchful eye of researchers Adrian Drazic, Henriette Aksnes and Michael Marie of the Arnesen lab and their international collection of colleagues, who joined forces to not only uncover the treasure of actin’s NAT, but also generated findings that shed considerable light on our understanding of cytoskeleton assembly and cell motility. These results have important implications for the possible development of new therapeutics, aimed at treating diseases where cellular hypermotility can be a real problem, such as cancer metastasis.
The hunt for actin’s N-terminal acetyltransferase points to NAA80
The abundance of actin in the human body attests to the importance of this protein for cellular functions, and if you want to find the reason why, then “follow the money trail.” The activity of actin is regulated by actin-binding proteins (ABPs) as well as posttranslational modifications, of which Nt-acetylation is a highly conserved process, with an emergent key role in the regulation of cell functions, protein interactions, and folding. A good suspect for this hunt was Nat6/FUS-2/NAA80 (NAA80), previously described by Zegerman et al., which is considered to be an N-terminal acetyltransferase (NAT). To investigate NAA80 further, Nt-acetylation assays were performed on NAA80 isolates from HeLa cells and recombinantly-expressed NAA80 against a peptide panel with various N-termini. NAA80 was found to Nt-acetylate both β- and γ-actin. Further, two HAP1 knockout cell lines (KO1 and KO2) were generated via CRISPR/Cas9 to hunt for NAA80 substrates. Further analysis confirmed that the substrate was indeed, actin– at last, the “cellular Titanic” had been found!
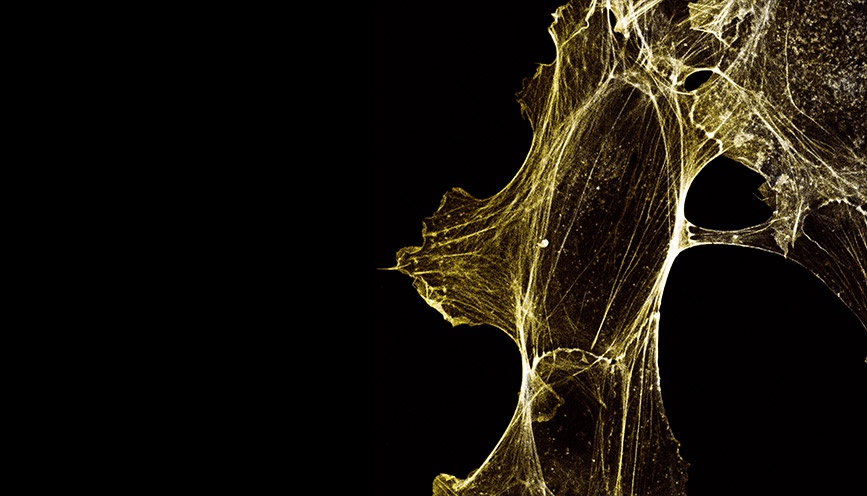
Characterizing the role of NAA80 on cell motility and cytoskeleton morphology
The next step was to assess the role of Nt-acetylation on the cytoskeleton, and how this impacts cellular motility. Along with other methods, IncuCyte® Live-Cell Imaging and Analysis of wound healing and chemotaxis were performed in the NAA80 knockout cell lines KO1 and KO2. We touched base with Dr. Henriette Aksnes, who performed live-cell imaging and analysis using the IncuCyte® system for these studies, to share her perspective on the use of this technology in her workflow.
How was IncuCyte® Live-Cell Imaging and Analysis used in your assessment of actin Nt-acetylation on cell motility and chemotaxis, and what new insights were revealed?
“Just as we had discovered that actin was NAA80’s substrate, and therefore wanted to look into cytoskeletal phenotypes, an IncuCyte® became available at our imaging facility, Molecular Imaging Center (MIC). This was very timely for us! I used the scratch wound maker to perform wound healing analysis on the HAP1 CTRL, NAA80 KO1, and NAA80 KO2 cells. With the 96-well setup, we got nice statistics and could show that the cells lacking NAA80 – and therefore the N-terminal acetylation modification of actin – actually closed the wound faster. We wondered whether this would also be the case for single cell migration, so we set up a chemotaxis assay. Here we used the chemotaxis plates that contains a transparent membrane with pores that cells can migrate through. I thought it was a very good idea that migrated cells could be distinguished in the image analysis by appearing in focus at the bottom of the membrane. We simply used higher FBS concentration as attractant, and found that “motivated” single cell migration was also increased in the NAA80 KO cells lacking actin Nt-acetylation. Even in wells where there was no FBS gradient as attractant, the NAA80 KO cells passed through the pores more frequently, so we concluded that random migration was increased as well. I could later confirmed with single cell trajectory plots. “
What effects did actin Nt-Acetylation have on cytoskeletal morphology?
“By phalloidin staining fixed cells we could examine the actin cytoskeletal fibers in detail. It was clear that the hypermotile NAA80 KO cells also showed cytoskeletal morphologies corresponding to increased locomotion activity, such as increased presence of membrane protrusive structures like lamellipodia and filopodia.”
How did actin Nt-acetylation affect actin polymerization and stability?
“Together with our collaborators at University of Pennsylvania, we were able to purify actin from the NAA80 KO vs. CTRL cells and could therefore compare the polymerization properties in vitro. These results showed that both elongation as well as depolymerization rates were reduced. This suggested that the filament turnover dynamics might be altered to potentially cause more stable filaments.”
How might these findings potentially impact the study of diseases where cellular motility is altered and possible therapeutic development?
“The hypermotility phenotype, combined with knowing that the NAA80 gene is located at a tumor suppressor region, motivated us to advance our migration studies to Matrigel® 3D cell culture invasion assays that are currently ongoing. If NAA80 turns out to be a biomarker for cancer metastasis, this could have implications for choice of treatment as well as revealing a new potential drug target.”
We sincerely thank Dr. Henriette Aksnes and her esteemed colleagues for their hard work and dedication, which helps to solve an important mystery of biology. We also congratulate this group on being awarded “Papers of the Year in 2018” from the University of Bergen, Faculty of Medicine. Well done!
Watch a video of wound closure from this study, captured and analyzed with IncuCyte®.
Learn more about IncuCyte® Scratch Wound Migration and Invasion Assays.
Related Publications:
Drazic A, Aksnes H, Marie M, et al. NAA80 is actin's N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility. Proc Natl Acad Sci U S A. 2018, Apr 24;115(17):4399-4404. Read More
Goris M et al. Structural determinants and cellular environment define processed actin as the sole substrate of the N-terminal acetyltransferase NAA80. Proc Natl Acad Sci U S A. 2018 Apr 24;115(17):4405-4410. Read More
Zegerman P, Bannister AJ, Kouzarides T. The putative tumour suppressor Fus-2 is an N-acetyltransferase. 2000, Oncogene 19:161–163 Read More
Aksnes H, Ree R, Arnesen T. Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases. Mol Cell. 2019 Mar 21;73(6):1097-1114. Read More
Dr. Henriette Aksnes, UiB–Department of Biomedicine, University of Bergen, Bergen, Norway
Photo by Renato Straume Fogliani

