- Home
- Applications
- Measure Cell Migration and Chemotaxis in Real Time
- Incucyte® Chemotaxis Assay
- Optimizing the IncuCyte® Chemotaxis Cell Migration Assay
TECH TIPS
Optimizing the IncuCyte® Chemotaxis Cell Migration Assay
Cells in the IncuCyte® Chemotaxis Cell Migration assay are required to move towards a chemoattractant across the membrane surface and through a pore. As a result, cells must be maintained in a healthy state, and the surface must be biologically relevant in order to facilitate cell movement. This often requires media conditions and surface coatings that are not required in the conventional Boyden chamber assays where cell health and biologically relevant surfaces are less important to assay performance. Assay medium formulations, cell surface coatings, cell density and starvation conditions are parameters that should be optimized in order to achieve superior assay performance.
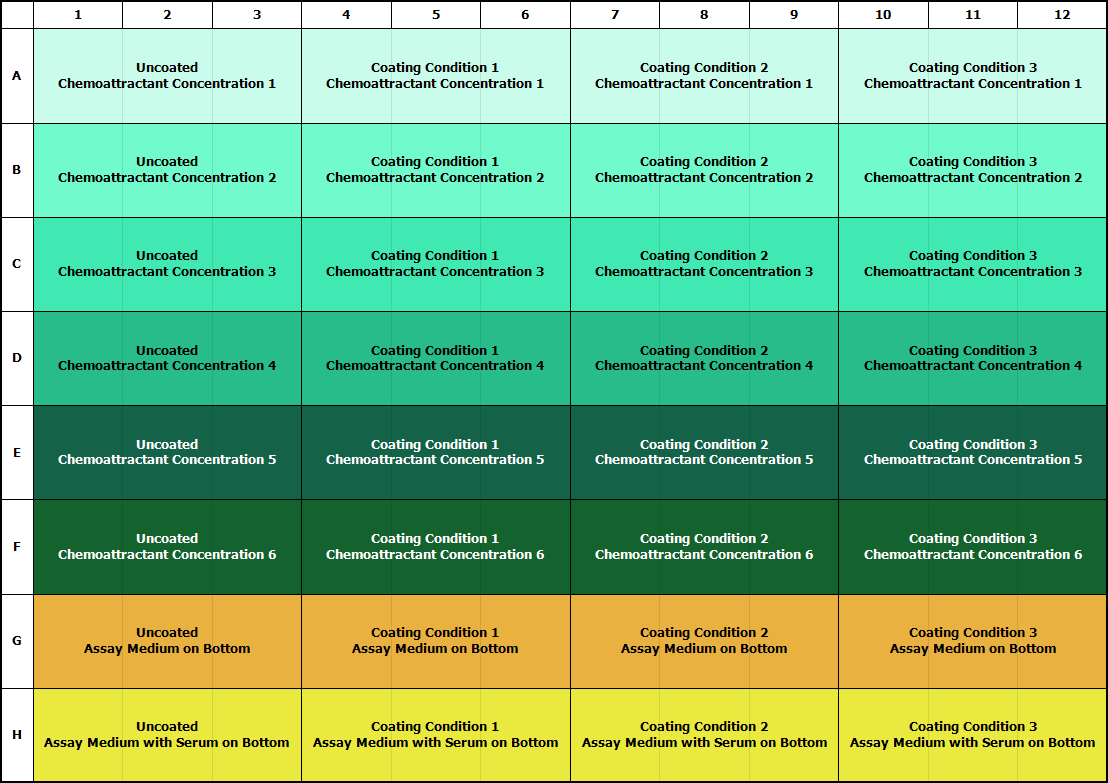
Surface Coating
Migrating cells require interactions with the substrate in order to move. Different cell types require different substrates. We commonly test collagen I, collagen IV, gelatin (attachment factor), fibronectin, Matrigel®, and serum as surface coatings, each at two to four different concentrations. Many cell types (e.g., neutrophils, T cells) will not move on an uncoated membrane. Learn how to coat ![]()
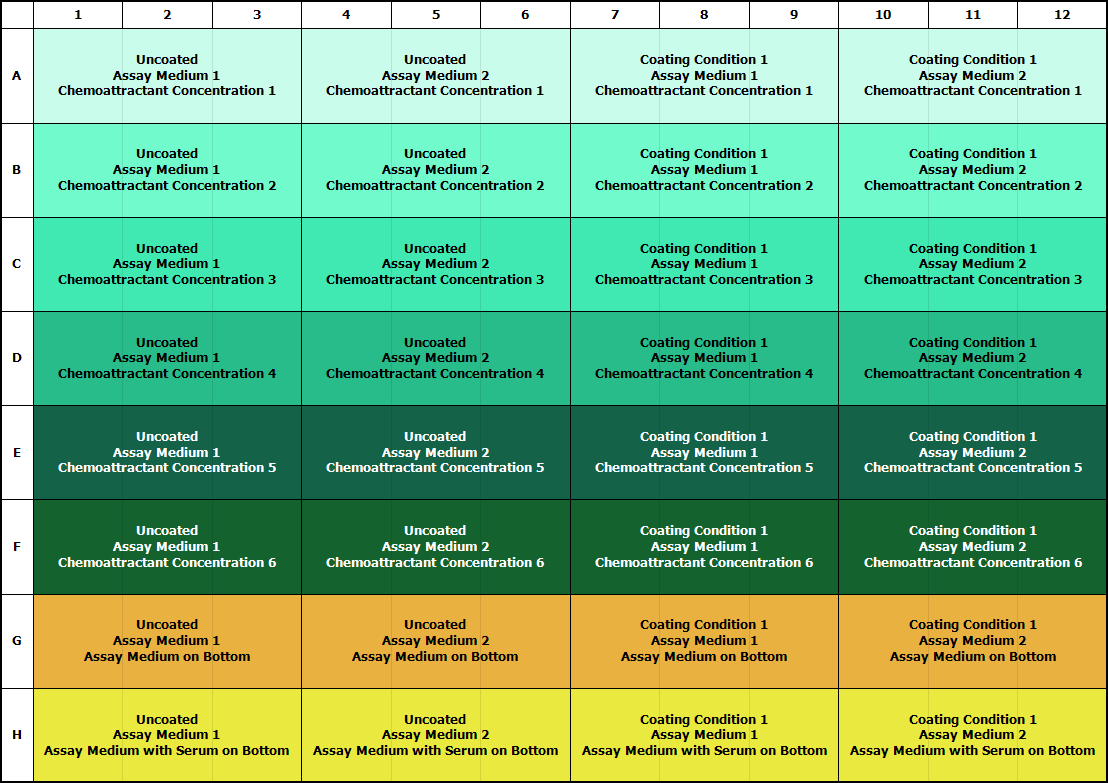
Medium
We have found that including a reduced amount of serum (0.1%–2.5%) in the assay buffer allows the cells to attach to the surface and move, while not affecting their directional migration. If cells look unhealthy, an experiment should be designed to increase serum or growth factor levels until cells are healthy enough to attach and move. Adding Insulin-Transferrin-Selenium (ITS) to the assay medium is another way to make cells healthier if minimizing serum is required.
Starvation
Serum starving cells prior to assay setup is a common practice in traditional Boyden chamber protocols. However, we have found that starving cells is not always required, and can be detrimental to cell health. For cell types that do require a significant starvation period in order to directionally migrate we recommend using the medium supplement Insulin-Transferrin-Selenium (ITS) during the starvation period.
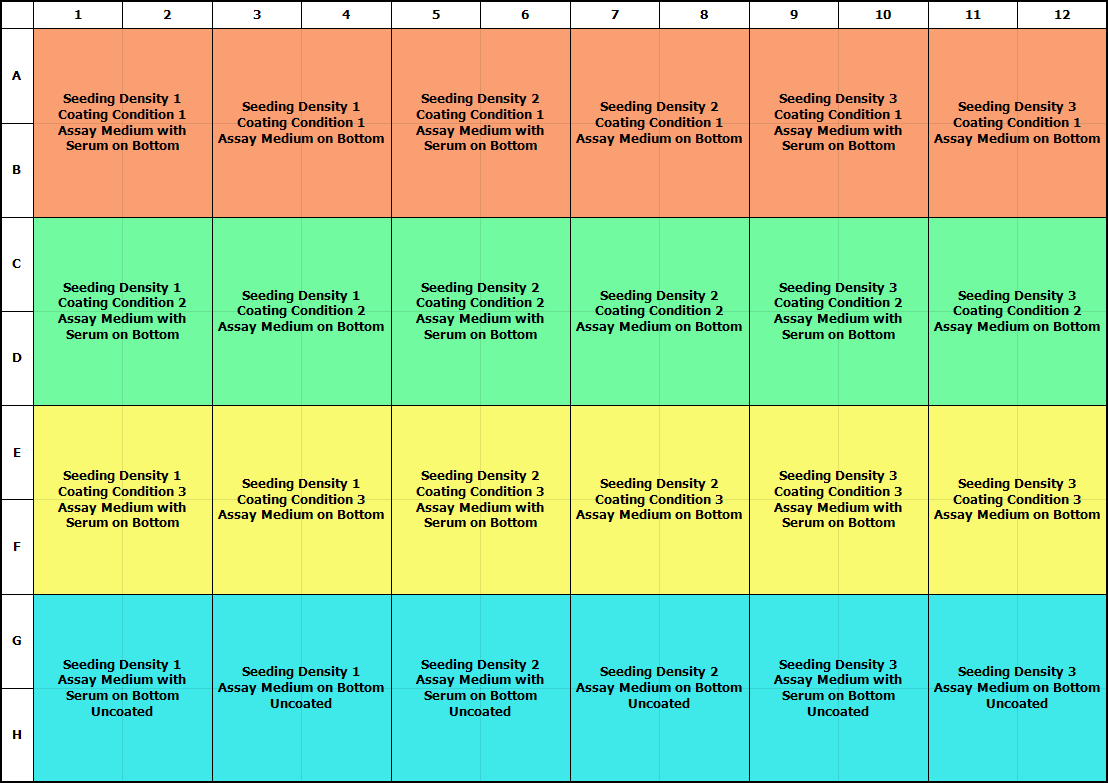
Cell Density
Some cells are the at their healthiest when they have other cells nearby. A cell density dilution series is recommended to optimize performance. Depending on cell size, a good range to start with is 1,000 to 5000 cells per well. Care should be taken to avoid extremely high cell densities, as this may cause issues with accurate masking of cells using the integrated software analysis tools.
Chemoattractant
The chemoattractant concentration required for an optimal assay should be determined through experimentation. Based on published literature and/or experience, we recommend completing a 6-point agonist curve starting with concentrations 10 to 20 times higher than the reported concentrations and using 3-fold dilutions. This should put the expected concentration near the middle of your curve and will give you an indication of the optimal agonist concentration.
Prior to starting optimization assays, we recommend a thorough review of literature in order to become familiar with standard culturing techniques as well as Boyden chamber assay conditions for the cell type of interest. Although not all techniques will translate into the IncuCyte® Chemotaxis Cell Migration Assay, they will give guidance for an optimization strategy. Usually, the above factors can be examined by outlining a defined approach that will allow optimization to be completed with two to three 96-well plates.
Strategies/Example
- Test chemoattractant and different types of extracellular matrices (ECM) first. We recommend using an assay medium containing 0.25% FBS to maintain cell health, as well as an initial seeding density of 1,000-5,000 cells per well.
- We recommend performing a 6-point agonist curve (3-fold dilutions) with maximal concentration 10 to 20 times higher than the reported EC50 value.
● Test a single concentration of each ECM. The concentration chosen should be based on literature information and your knowledge of the cell type. If only one ECM is available, test multiple concentrations.
● You will be able to evaluate cell health by using the IncuCyte® phase-contrast images to inspect the cell morphology.
● Post-results, select ECM and further evaluate the concentration required in the assay (if required). - Fine-tune the assay medium and coating parameters.
● Prepare different assay media containing FBS (0.1%-2.5%), or remove serum completely. We have found that the addition of supplements, such as ITS, may be required to maintain cell health.
● Coat the top and/or bottom surfaces with one to four concentrations of the previously identified ECM.
Once medium, starvation (if required), coating (if required), and cell density parameters are understood, perform a 7- to 11-point concentration curve to determine maximal response of the cell type to the chemoattractant. Refer to the plate maps below for optimization approaches.