- Home
- Applications
- Measure Cell Migration and Chemotaxis in Real Time
- Chemotactic Transendothelial Migration

Incucyte® Applications
Chemotactic Transendothelial Migration Assay for Live-Cell Analysis
What is transendothelial migration?
Transendothelial migration is the movement of leukocytes across the endothelium at the site of inflammation and is a critical step in the immune response. Assays to evaluate the extravasation of leukocytes are essential for the study host-defense response and inflammatory disorders.

4th Edition! Live-Cell Analysis Handbook
A guide to real-time live-cell imaging & analysis

Application Note:
Gain additional insights into the mechanism of immune cell killing by combining live cell analysis and flow cytometry into a single workflow
Download Your CopyOverview
Introducing the Incucyte® Chemotaxis Transendothelial Migration Assay
An integrated solution for real-time visualization and automated analysis of transendothelial migration in 96-well format.
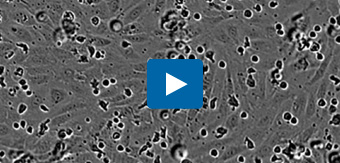
Visualize transendothelial migration: leukocyte extravasation across an endothelial cell monolayer. Directed migration of primary T cells through a monolayer of HUVEC endothelial cells toward the chemoattractant SDF-1α, using an Incucyte® Clearview 96-well Plate. Cells were visualized and quantified in real time using the Incucyte® Live-Cell Analysis System. Integrated Incucyte® Chemotaxis Analysis Software Module automatically quantifies the loss of leukocytes from the upper membrane surface as they move through the endothelial layer.
Chemotaxis Transendothelial Migration Quick Guide

Key Advantages
Key advantages of the Incucyte® Transendothelial Migration Assay
- Visually assess monolayer integrity and leukocyte diapedesis
- Automated analysis of transendothelial migration
- Investigate treatment effects on adhesion and migration of leukocytes
- Assay compatible with multiple cell types
Visually assess monolayer integrity and leukocyte diapedesis
- Ensure that the endothelial monolayer remains intact throughout your experiment
- Visualize leukocyte crawling and diapedesis through intact endothelium
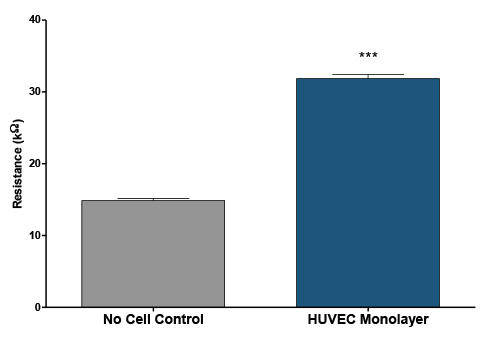
Conductivity Testing of transendothelial migration. Resistance measurements of a Human Umbilical Vein Endothelial Cell (HUVEC) monolayers cultured on a supporting matrix of fibronectin was measured using a digital multimeter. HUVEC monolayers were grown for 24 hours in EGM-2, then growth medium was removed and 60 µL of DPBS -/- was placed into the insert wells and 200 µL into the reservoir wells. The average resistance for inserts containing a monolayer was 31.9 ± 1.7 compared to wells without cells 14.9 ± 0.9 (N=9 per condition), indicative of monolayer formation over the insert pores.
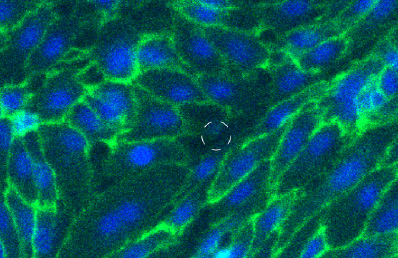
Visualize transendothelial migration using immunofluorescence staining. HUVECs were seeded at 6,000 cells per Incucyte® Clearview insert and allowed to form a monolayer for ~24 hrs. Cells were permeabilized and fixed, and VE-cadherin adhesion junctions (green) were visualized from assembled 10x Z-stack confocal images (dashed circles mark membrane pore location). Images show intracellular contact integrity of HUVEC monolayer, which is vital for the control of leukocyte extravasation.
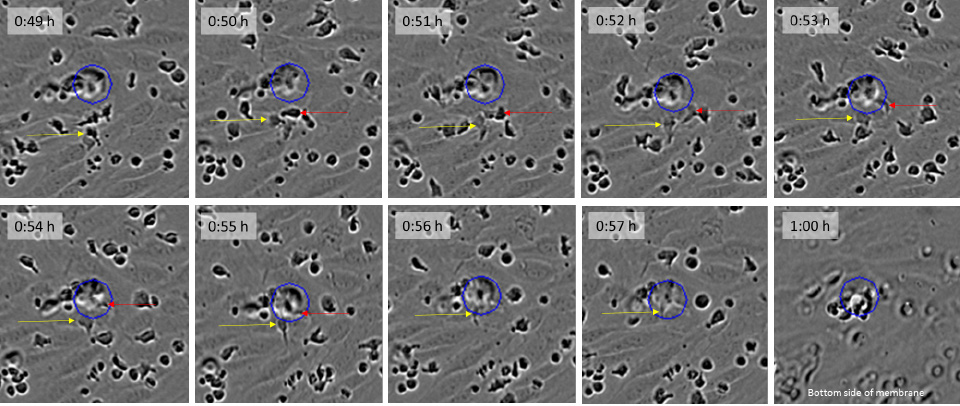
Visualization of leukocyte extravasation. CD3/CD28 Dynabead activated primary T cells were seeded on a HUVEC monolayer cultured on fibronectin and images were acquired every minute using the Incucyte® Live-Cell Analysis System. Yellow and orange arrows indicated leukocytes moving between HUVEC cells and eventually down the pores (blue circles) of the Incucyte® Clearview insert.
Automated analysis of transendothelial migration
- Fully quantify directional cell transmigration with no manual cell counting
- Label-free, kinetic monitoring of transendothelial migration
- Set up and walk away – fully automated image based quantification
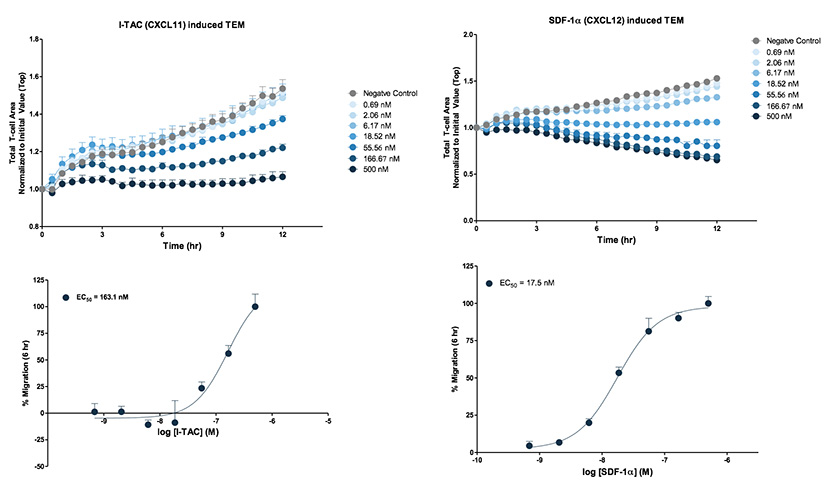
Primary T cells extravasation toward CXCL11 and CXCL12. CD3/CD28 Dynabead activated primary T cells were seeded on a HUVEC monolayer cultured on fibronectin. The insert containing T cell:HUVEC monolayer co-cultures was then exposed to either CXCL11 or CXCL12 gradients at the indicated concentrations. Images were acquired every 30 minutes using the Incucyte® Live-Cell Analysis System and phase analysis was performed. Analysis of pharmacological response was performed at t=6 hr. Each data point represents mean ± SEM, N=4.
Investigate treatment effects on adhesion and migration of leukocytes
- Characterize pathways involved in transendothelial migration through pharmacological profiling and genetic manipulation
- Measure transendothelial migration in up to six 96-well plates at once
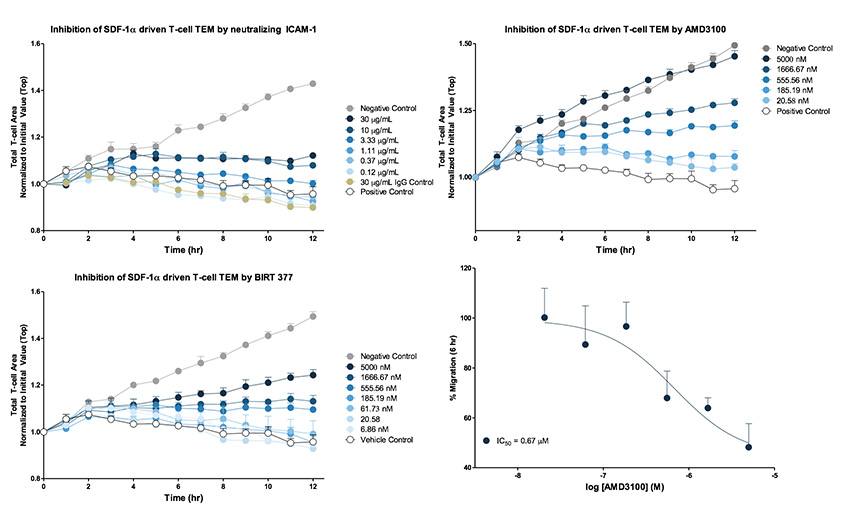
Inhibition of leukocyte extravasation. For antibody inhibition, HUVEC monolayers were cultured overnight on fibronectin and then pre-treated with neutralizing ICAM-1 for 1 hour at 37°C. CD3/CD28 Dynabead activated T cells were plated at a density of 5,000 cells per well on to the HUVEC monolayer then exposed to 100 nM SDF-1α (EC80 concentration). For BIRT 377 (allosteric modulator of LFA-1) and AMD 3100 (CXCR4 receptor antagonist) inhibition studies, CD3/CD28 activated T cells were pre-treated for 1 hour at 37°C at indicated inhibitor concentrations, then seeded onto HUVEC monolayers and exposed to 100 nM SDF-1α. Phase contrast images were collected every hour over a 12 hour period and analysed. Analysis of pharmacological response was performed at t=6 hr. Each data point represents mean ± SEM, N=3.
Assay compatible with multiple cell types
- Validated with primary T cells, neutrophils and Jurkats
| Leukocyte | Endothelial Cells | Endothelial Growth Medium | Assay Medium | Coating (20 µL top/150 µL bottom) |
Seeding Density endothelial: leukocyte (cells/well) |
|---|---|---|---|---|---|
| Jurkat | HUVEC | EGM-2 | EBM-2 + 2% FBS with #supplements | 5 µg/mL Fibronectin or 50 µg/mL Collagen | 6,000 : 5,000 |
| Primary T cells | HUVEC | EGM-2 | EBM-2 + 2% FBS with #supplements | 5 µg/mL Fibronectin or 50 µg/mL Collagen | |
| EA.hy926 | DMEM + 10% dialyzed FBS | RPMI + 0.5% dialyzed FBS | 50 µg/mL Collagen | ||
| Neutrophils | HUVEC | EGM-2 | EBM-2 + 2% FBS with #supplements | 5 µg/mL Fibronectin or 50 µg/mL Collagen |
# Supplement includes gentamycin, hydrocortisone, ascorbic acid
Chemotaxis FAQs
Membrane-based transwell systems for chemotaxis assays are capable of maintaining chemoattractant gradients for a few hours, but beyond that they collapse, leading to equimolar amounts of chemoattractant on both sides of the membrane. ClearView 96-Well Chemotaxis Plates rely on 96 tiny pores (8 µm in diameter) to set up and maintain the chemoattractant gradient for 72 hours. Since chemotaxis migration assays rely on chemical gradients to differentially induce migratory behavior in cells, maintaining the chemoattractant gradient is essential.
Yes, rather than just citing the reduction of cells on the surface of the membrane, the IncuCyte™ ZOOM can provide real-time analysis of the movement of cells through the pores to the underside of the well. It simultaneously provides live-cell analysis of both surfaces of the insert, enabling morphological analysis of the cells before, during, and after migrating through the pores.
Chemotaxis assays analyze cell migration in response to a chemoattractant, a process that guides cell trafficking in vivo. Examples of chemotaxis assay applications include the analysis of chemotactic migration across substrate surfaces, chemotactic invasion through 3-D biomatrix gels, and chemotactic transendothelial migration of leukocytes (extravasation or diapedesis). Studying chemotaxis requires the generation of chemotactic gradients, as cells travel from areas with one concentration to areas of another (high concentration to low concentration, or low concentration to high concentration). Some chemotaxis assays rely on membranes to maintain these chemoattractant gradients, but those gradients are unstable and they rapidly collapse. ClearView 96-Well Chemotaxis Plates enable gradient maintenance for at least 72 hours -- Something other chemotaxis assay systems can’t claim.
Learn more about the ClearView method for chemotaxis assays
Leukocyte extravasation is the process by which leukocytes in the circulation stop rolling along vascular endothelium and migrate across the endothelium in response to inflammation. In order for any system to accurately recapitulate transendothelial migration, it must first involve the generation of a monolayer of endothelial cells over top of the pore-based insert. Once confluence of the endothelial cells is established, the leukocytes can be carefully added to the culture, where they will extravasate between the endothelial cells in response to the chemoattractant in the reservoir.
View the IncuCyte™ transendothelial migration assay protocols
The most common effector cells used in the study of trans endothelial migration of leukocytes in vitro include primary T cells, neutrophils, and Jurkat cells. The most commonly used cells to recapitulation the endothelial cell layer are HUVECs. Other cells not listed here that exhibit the ability to extravasate through the endothelium may work with this system, but the protocol – including the choice of endothelial monolayer – will need to be experimentally determined.
While immune cells are non-adherent, their interaction with ECM on the substrate is essential for their migration. Immune cells do not migrate on uncoated surfaces, but can readily migrate in the presence of appropriate ECM proteins. Examples of substrate coatings that are compatible with immune cell chemotaxis include ICAM-1, fibronectin, and Matrigel®/FBS.
Ordering info
Incucyte® Chemotaxis Analysis Software Module
An add-on software module for the Incucyte® Live-Cell Analysis System. Analyze label-free and fluorescently-labeled chemotactic cell migration images acquired using the Incucyte® Clearview Plate. This software is required for analyzing chemotaxis using the Incucyte® Live-Cell Analysis System.
Incucyte® Clearview 96-Well Plate for Chemotaxis
The Incucyte® Clearview 96-Well Plate provides an optically clear surface for label-free imaging and analysis of chemotactic cell migration within the Incucyte® Live-Cell Analysis System. The plates are required for analyzing chemotaxis using the Incucyte® Live-Cell Analysis System.
Tech Resources
Technical Resources
Posters
Documentation
- Incucyte® Cell Migration & Invasion Brochure
- Incucyte® Chemotaxis Application Note
- Incucyte® Chemotaxis Transendothelial Migration Assay Protocol
- FAQ